
The Mouse Behavior Laboratory, a CIDD core facility, provides investigators within the IDDRC and throughout the UNC research community with a wide variety of behavioral tasks for studies in genetic, environmental, and pharmacological models of human disorders, and for preclinical efficacy testing of novel therapeutic agents. The Laboratory includes state-of-the-art equipment for the measurement of mouse phenotypes, and offers training and consultation regarding the utilization of rodent models. Available testing regimens include a standardized battery for measures of general health and neurological reflexes, procedures for sensory and motor abilities, and evaluations of anxiety-like behavior, social interaction, sensorimotor gating, cognitive function, and abnormal repetitive behavior.
Project Manager: Kathryn Harper, Ph.D.
Behavioral Tests
All behavioral testing procedures are conducted in strict compliance with the policies on animal welfare of the National Institutes of Health (stated in the "Guide for the Care and Use of Laboratory Animals," Institute of Laboratory Animal Resources, National Research Council, 1996 edition), and are approved by the University of North Carolina Institutional Animal Care and Use Committee.
Motor function

-
Open field activity and exploration. Locomotion, fine movements, rearing, and center time are measured using
automated activity systems (Fusion and VersaMax Systems, AccuScan Instruments). These data can be used to
quantitate hyper- or hypo- activity, anxiety-like behavior, and stimulant or sedative drug effects.
-
Grip strength. Paw grip function can be evaluated using digital force meters (San Diego
Instruments) or in a wire hang test.
-
Accelerating rotarod test for motor coordination and balance. Mice are placed on a cylinder which slowly accelerates to a constant rotating speed. Normal mice readily learn to walk forward as the drum turns.
-
Swimming. A video tracking system (Ethovision, Noldus) is used to measure swimming ability in a water maze.

Sensory tasks
- Olfaction is evaluated by scoring the retrieval of a buried food.
- Hearing is tested using an automated acoustic startle procedure (SR-Lab system, San Diego Instruments).
- Vision is tested with the visible platform task in a water maze or by a visual cliff procedure.
- Thermal sensitivity is tested with a hot plate/cold plate apparatus (IITC).
Tests for anxiety- and depression- related behavior
- Open field. Time spent in the center region is used as an index of anxiety-like behavior.
- Elevated plus maze or zero maze. Mice are given a choice between staying within the safety of two walled areas, versus exploring two open areas.
- Light/dark exploration. Mice are given a choice between staying in the dark side of a chamber, or entering the lighted side (Versamax, Accuscan).
- Forced swim. Measures are taken of time spent immobile by an image tracking system (Ethovision, Noldus).
- Sucrose preference: Home cage drinking is monitored during a two-bottle choice test.

Social behavior
- Three chamber choice test for sociability and preference for social novelty
- Direct social interaction during play. Aggression in a resident-intruder assay.
- 5-trial test for social recognition.
Learning and memory tasks
- Morris water maze: This is a standard assay for acquisition of spatial learning and cognitive flexibility during reversal learning.
- Barnes maze: This apparatus provides indices of spatial and reversal learning similar to the water maze, but without the physical demands of swimming.
- Y-maze: Working memory in a test of spontaneous alternation.
- T-maze: Spatial learning, alternation, and reversal can be tested in a T-maze, using food or liquid reinforcers. A delayed non-match-to- sample procedure can be utilized to demonstrate working memory deficits.
- Conditioned fear and avoidance tests: The core uses a Near-Infrared
Video Fear Conditioning system (Med Associates) for evaluating contextual or cue-dependent learning.
- 5-choice serial reaction time task (5-CSRTT). An operant test for learning, visual attention, vigilance, and response inhibition (Med Associates).

Sensorimotor gating
The acoustic startle test (San Diego Instruments) is used to evaluate auditory function and sensorimotor gating. The test is based on the reflexive whole-body flinch, or startle response, that follows exposure to a sudden noise. Prepulse inhibition, a measure of sensorimotor gating, occurs when the addition of a softer noise, or prepulse, immediately before the loud startle stimulus, leads to a significant inhibition of the subsequent startle response. Deficits in prepulse
inhibition are associated with schizophrenia, autism, and other neuropsychiatric disorders.
Repetitive behavior
The core can assess abnormal repetitive behavior, restricted interests, and stereotypy using several different approaches, including reversal learning in the water maze, digging in a marble-burying task, rates of grooming, jumping, or other behaviors (scored using
Observer software, Noldus), and exploration during an automated nose-poke task (Pokeman, Accuscan Instruments).

Neonatal assessment
Young mice can be evaluated with measures of ultrasonic vocalization (UltraVox, Noldus, or Avisoft recording systems), activity in a Phenotyper (Ethovision, Noldus), prepulse inhibition of acoustic startle responses, and social approach, coded using Observer software (Noldus).
Laboratory User Information
Services. The core offers expert testing services, including the characterization of mutant mouse lines across multiple domains of function. In addition, the core can provide consultation on study design for projects or grant applications, training on techniques for phenotyping, and access to the behavioral testing equipment and facility.
Project initiation. UNC investigators wishing to utilize the core should contact the director, Dr. Sheryl Moy, ssmoy@med.unc.edu, to discuss optimal study designs, feasibility for core use, and costs of testing. All projects utilizing the core must be approved by the UNC Institutional Animal Care and Use Committee (IACUC) before initiation. The director can provide drafts of amendments or sections to include behavioral testing in animal protocols.


Core charges. Costs are based on personnel effort for conducting studies, including testing time, set-up and clean-up, and data transformation and analysis. As a component of the UNC IDDRC, the Laboratory receives significant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Therefore, the core can provide free pilot experiments and subsidized rates for IDDRC investigators. Please contact the director for project cost estimates.
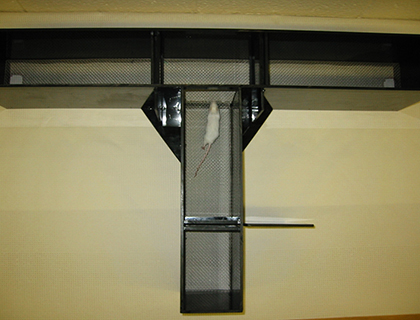
Transfer of mice to the core facility. The Mouse Behavior Laboratory is located in the Neuroscience Research Building (NRB), inside the Division of Comparative Medicine (DCM) animal vivarium. The laboratory consists of 6 behavioral testing rooms, and an adjacent mouse housing room (1116 NRB). All transfers of mice to the Core must first be approved by DCM. Because the NRB vivarium is a closed animal facility that maintains maximal cleanliness, only mice from other UNC facilities at a similar health status, or from commercial sources such as Jackson Laboratory, may be transferred to core housing. Rederivation may be required for mice that do not meet the DCM health standards for the NRB vivarium. Mice imported from other academic institutes with high health standards must first undergo a 6-9 week quarantine period before transfer to the core, dependent upon DCM approval.

For each transfer group, the director will need an Excel spreadsheet with information about the mice, including subject ID, genotype, sex, DOB, and DCM cage card number. Because single housing is stressful for mice, the core asks that each cage contain from 2-4 mice, separated by sex. For most projects, a maximum of 24 mice can be transferred to the core at one time
Experimental rigor and reproducibility. The Behavior Laboratory provides consultation on study design to address issues of rigor, including use of littermate controls, consideration of sex, inclusion of appropriate subject numbers, balancing across cohort groups, and randomization of subjects to treatment conditions. Experiments in the laboratory are conducted by researchers blind to genotype, treatment, or other study variables. Statistical analyses include standard parametric approaches for data that are normally distributed, with appropriate post-hoc tests and consideration of multiple comparisons. In the case of non-normal data, nonparametric rank-based regression or other appropriate analyses are used. Reproducibility of data is confirmed, in part, by comparing behavior in wild type mice across projects and archived strain distributions and mutant line profiles. In addition, sets of C57BL/6J and other strains are periodically evaluated in the laboratory to ensure that assays are providing the expected indices for typical performance.
Guidelines from the UNC Office of Research Technologies
A. Eight steps to Rigorous and Reproducible Experiments at UNC:
- If using a core facility, consult with the core staff in the planning stage. Consult with a statistician if you need help developing a Power Analysis to assure that your results will be adequately powered.
- Design your experiment with sufficient controls (rigor) and replicates (reproducibility).
- Assure that ALL of your reagents (antibodies, cell lines, mice) are fully validated.
- Have a clear and detailed protocol (SOP) and data analysis plan. Assure that the protocol is strictly followed or that any deviation is well documented.
- Assure that the staff or students performing the experiment are well trained and understand each step and the importance of performing them precisely.
- Use only well-maintained instrumentation, preferably maintained and operated in a core facility with expert staff (see #1 above).
- Document all steps, reagents, equipment and data analysis methods used in the experiment. Assure that the both the documentation and the data itself are properly stored in a safe data management repository.
- Acknowledge grants that support the research, including cores (by name) and core staff, in publications.
B. Guide to Rigor and Reproducibility for the Mouse Behavioral Phenotyping Laboratory
- Consult with the core director in the planning stage:
Sheryl S. Moy, Ph.D.
ssmoy@med.unc.edu
- Subject numbers. Mouse behavior studies typically require 10 to 12 subjects per experimental group for adequate power in the statistical analyses. Thus, for a line of genetic mutants, an N of approximately 10 wild type and 10 mutant littermates is required. To evaluate effects of both sex and genotype, approximately 10 males and 10 females of each genotype will be required.
- Ensure the use of appropriate controls for behavioral studies. In many cases, the optimal controls are wild-type littermates of mutant mice, derived from heterozygous breeding pairs. Biological variables that require consideration include age, sex, and genetic background. Other important factors are source of the mice (i.e. from a commercial laboratory or bred in-house), environmental conditions, and experimental history.
- Housing conditions can affect mouse behavior. In general, mice for testing in the core should be group housed, with a maximum of 4 per cage. Individual housing should be avoided, since isolation can be stressful, especially for younger mice. Exceptions can be made for males separated for fighting or other reasons related to health, or when isolate housing is required for the experiment.
- Acknowledgements. All publications and presentations that include data generated using core resources should acknowledge support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; P50 HD103573).
- Co-authorship of core faculty and staff is dependent on the significance of their contribution to the research. Please consult the website of the Association of Biomolecular Resource Facilities for policies on the appropriate inclusion of Core Laboratory Personnel in authorship of your publication.
https://abrf.org/authorship-guidelines
To be considered for membership in the CIDD and to gain access to core resources of the UNC IDDRC, please visit the Membership and Access Information page.
Membership and Access Information
Mouse Behavioral Phenotyping Laboratory Contact Information
Sheryl Moy, Director
Phone: (919) 966-3082
Email:sheryl_moy@med.unc.edu
Mouse Behavior Laboratory
Phone: (919) 966-8651
Email: Viktoriya Nikolova Viktoriya_Nikolova@med.unc.edu
Office Address
4200 Medical Biomolecular Research Building (MBRB) 111 Mason Farm Road
Campus Box 7146 UNC-CH
Chapel Hill, NC 27599
